Summary: Biosimilars present unique development challenges, but the Mass Spectrometry-based Multi-Attribute Method (MAM) offers a deeper understanding of molecule attributes by analyzing multiple factors in a single workflow. This advanced technique ensures efficient analysis, reduces variability, and maintains the consistent quality and safety of biosimilars.
Biological medicines (biopharmaceutical) have transformed the way we treat chronic diseases such as cancer, diabetes, and autoimmune disorders, leading to remarkable improvements in patient outcomes. However, biologics can be expensive, limiting access for many patients.
In this scenario, biosimilars offer affordable, high-quality alternatives, potentially reducing healthcare costs and improving access to essential therapies.
However, the development of biosimilars presents a significant challenge: understanding and controlling molecular variations that naturally arise because of the manufacturing process and complex nature of the biological molecule itself. By studying these molecular differences, scientists can ensure biosimilars work just as effectively as the original biologic.
At the 5th Annual Summit on Biopharmaceutical Development in Goa, India (2025), Dr. Anita Krishnan, Associate Vice President & Head of Analytical Sciences at Biocon Biologics, provided critical insights into this issue. In her talk, “The Eternal Conundrum of Peaks and Attributes in Biopharmaceuticals,” she highlighted how analytical advances and innovation are transforming biosimilar development.
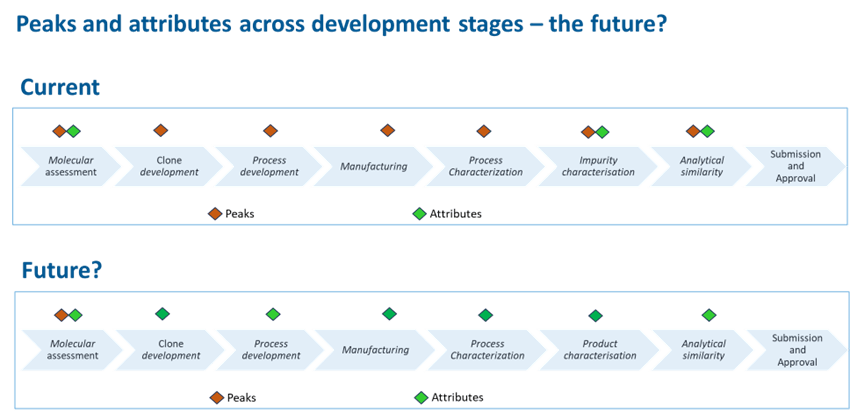
Unlike small-molecule drugs, which have well-defined and reproducible chemical structures, biologics are large, complex proteins produced in living cells, making them inherently variable. Due to this complexity, traditional analytical techniques such as chromatography and electrophoresis alone are not sufficient to determine the full biological impact of the inherent molecular variants on the overall function of the biologic. These techniques rely on the occurrence of specific molecular signals or ‘peaks’ to determine the specifics of molecular entities under consideration.
Peaks often consist of multiple attributes that represent different types of variants. For example, both deamidations and glycations are acidic variants. When these attributes overlap (co-elute), they may have different levels of importance, making it challenging to determine their biological impact.
In contrast, modern approaches, like mass spectrometry-based attribute analysis, identify specific molecular changes, offering clearer picture of how these variations affect biologic products behavior.
These techniques provide an in-depth look at molecular changes, allowing researchers to identify specific modifications in a molecule such as:
- Post-translational modifications (PTMs) or chemical changes occurring in a protein post- synthesis, such as :
- Glycosylation: It involves attaching sugar molecules to specific sites in a protein, which can impact its stability and how it interacts with other molecules.
- Oxidation: The addition of oxygen molecules, which can change the protein structure and function.
- Deamidation: The removal of an amide group, which can impact the protein’s stability and function. These modifications are crucial as they can significantly influence a biological product’s therapeutic properties.
- Charge variants, or variations in the protein’s charge due to modifications such as:
- Lysine truncation: The removal of lysine residues at the C-terminal end (part of the protein structure), can affect the protein’s stability
- Isomerization: a structural shift in the molecule that may influence protein’s stability and function.
- Size variants or differences in the size of the protein molecules, such as:
- Aggregates: Clusters of protein molecules that can form during production or storage, potentially leading to reduced efficacy or increased immunogenicity.
- Fragments: Smaller pieces of the protein that can result from degradation, which may impact the product’s safety and effectiveness.
Detailed understanding ensures that the developed biosimilars maintain consistent therapeutic properties, stability, and safety, leading to more effective treatments.
The Crucial Role of Analytical Advances in Shaping the Regulatory Landscape for Biopharmaceuticals
Global regulatory bodies are increasingly emphasizing on greater reliability of analytical assessments as a proof of biosimilarity. This has led to the adoption of advanced methodologies like:
- Multi-Attribute Method (MAM): A mass spectrometry-based approach that replaces multiple conventional tests with a single high-resolution analysis, as described in USP chapter <1060>.
- Separation Science Tools: High-performance chromatography techniques to resolve molecular variants.
Advantages of using MAM
- Meaningful specification setting: which helps in eliminating out-of-specification (OOS*) issues caused by attributes that are not-critical.
*OOS (Out-of-Specification) means that a product or material does not meet the required quality standards or limits during testing.
- Science-based comparability assessment: MAM (Multi-Attribute Method) can replace methods like CEX (charge variants), rCE-SDS (fragments), HILIC (glycan), HIC/RP (oxidation), and Identity methods, saving time, effort, and reducing the need for multiple instruments.
Challenges with the MAM
While the Multi-Attribute Method (MAM) offers significant advantages, its implementation comes with several challenges:
- High initial investment: The cost of instrumentation and software for MAM can be significant and limiting
- Bridging conventional methods: Transitioning from traditional analytical techniques to MAM requires additional effort and resources
- Potential loss of information: There is a possibility of not capturing all the detailed information about the protein’s modifications, which may impact the overall quality assessment of the biopharmaceutical
- Transitioning in-process testing controls: Adapting in-process testing controls to the MAM platform can be challenging due to slower turnaround times (TAT).
The Future of Biosimilar Development
As biopharmaceutical development moves towards science-driven accelerated pathways, there is a major shift in the industry from traditional analytical methods to high-resolution, mass spectrometry-driven attribute assessment and other multi-attribute methods. This shift ensures better process control, reduced product variability, and faster biosimilar approvals, ultimately improving patient access to affordable biologics.
Despite several implementation challenges, the potential benefits of MAM in ensuring the quality and consistency of biosimilars make it a promising tool for the future of biopharmaceutical development- potentially saving time and money by reducing the need for many clinical trials.
Biocon Biologics is actively utilizing MAM into its workflows to accelerate product development, ensuring faster, more efficient biosimilar development”
Authored by:
Anita Krishnan, Ph.D. (Biotechnology, Jawaharlal Nehru Technological University), Biocon Biologics R&D
Kavitha Rao, Ph.D. (Pharmaceutical Sciences, University of Nebraska Medical Center), Biocon Biologics Global Communications

