Biocon recently presented data from its Insulin Tregopil clinical program at the American Diabetes Association’s (ADA) 78th Scientific Sessions held at Orlando, Florida, from 22nd to 26th June, 2018.
The ADA Congress is one of the largest gatherings of diabetes researchers in the world, where scientists and healthcare professionals share and learn about new advances in diabetes with top experts. The future of insulin therapy was among the key research topics on the agenda at the annual ADA conference in Florida.
There are about 422 million people living with diabetes worldwide and about 40% of them rely on insulin, according to WHO.
People with diabetes who are dependent on insulin can currently be administered the hormone using a needle and syringe, a pen injection, a pump through a needle, and an inhaler.
As injections can be painful, most patients delay getting on to insulin therapy until it is very late. Moreover, insulin, when injected, is sometimes not as effective as it can be as it is administered into the peripheral blood stream, which does not mimic the way natural insulin works in the body.
With researchers having worked for decades to find a way to orally administer insulin to people with diabetes, an effective oral insulin molecule is now considered the ‘elusive’ Holy Grail of diabetes therapy.
The ability to deliver insulin through a pill will make it convenient for patients to take insulin, and hence improve compliance.
However, developing an oral insulin molecule that patients can ingest remains a scientific challenge because insulin, a peptide, is degraded in the gastro-intestinal tract by acids and enzymes before it reaches the liver and acts on the sugar levels.
The quest for a game changing insulin therapy led Biocon to invest in the clinical development of Insulin Tregopil, a first-in-class oral insulin molecule for post-prandial glycaemic control.
Insulin Tregopil is a modified form of human insulin in which a single short-chain amphiphilic oligomer is covalently linked by a non-hydrolysable amide bond to the free amino acid group on the Lys-β29 residue of recombinant human insulin.
The modification helps in:
- Improving the solubility over human insulin
- Stability of insulin polypeptide against enzymatic degradation in the digestive tract
- Systemic absorption of the intact peptide
These features make Tregopil different from other oral molecules, which get degraded in the gut.
Biocon’s oral insulin candidate:
- Mimics the physiological benefits of direct delivery of insulin into the portal system without sustained peripheral hyperinsulinemia
- Results in a restoration of the physiological gradient of insulin
- Minimizes the unwanted metabolic effects (e.g., weight gain; risk of hypoglycemia) due to systemic exposure
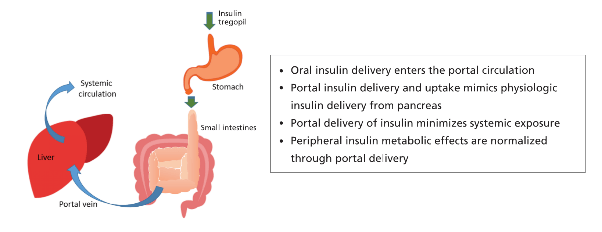
Initial studies in people with Type 2 diabetes as well as normal healthy volunteers have demonstrated an excellent safety profile for Tregopil, with evidence of significant post-prandial glucose excursion control in Type 2 diabetes patients. Biocon had announced successful results from the Phase I clinical studies on Insulin Tregopil that had concluded in the U.S. in 2016. These studies established the target product profile of this molecule, including food effects, drug-drug interaction and PK/PD profile.
Poster Presentation on Insulin Tregopil at #ADA2018
Dr Sandeep N. Athalye, Senior Vice-President, R&D at Biocon, made a poster presentation on the ‘Relative Dosing Time of Insulin Tregopil, a Novel Oral Insulin, and its Pharmacodynamic Effect in Type 2 Diabetes Mellitus Patients’ on June 24, 2018.
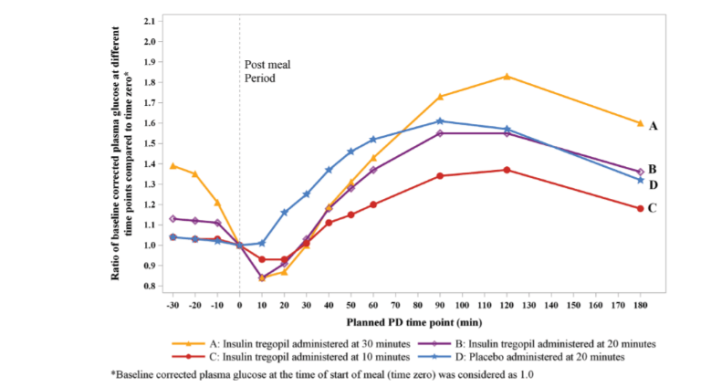
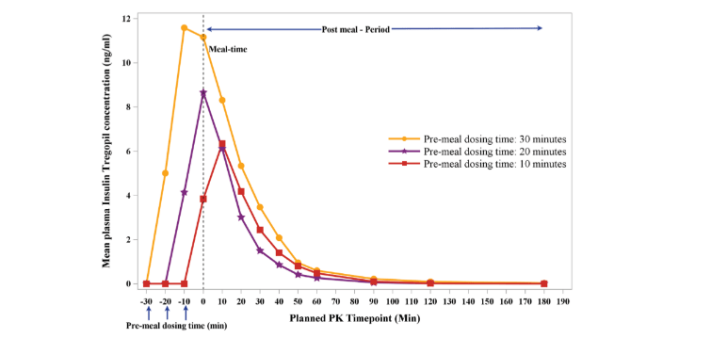
Dr Athalye’s poster presentation was based on the findings from a Phase I, randomized, placebo controlled, crossover study conducted in Type 2 diabetes patients at a single center in the U.S.

A total of 15 people with Type 2 diabetes were enrolled in this study.

Oral Presentation on Insulin Tregopil at #ADA2018
The poster presentation was followed by an oral presentation by Dr. Harold Lebovitz, an internationally recognized authority in the field of diabetes, on ‘Pharmacodynamic Effect of Novel Oral Insulin Tregopil in Relation to Meal Composition in Type 2 Diabetes Mellitus Patients’ on June 25, 2018.

Dr Lebovitz’s presentation was based on a Phase I, randomized, placebo controlled, crossover study conducted in Type 2 diabetes patients in the U.S. with Insulin Tregopil. A total of 18 people with Type 2 diabetes participated in this study.

The study showed that Insulin Tregopil with its rapid onset and ultra-short acting profile was safe and well tolerated when administered with various meal compositions in Type 2 diabetes mellitus patients on metformin.
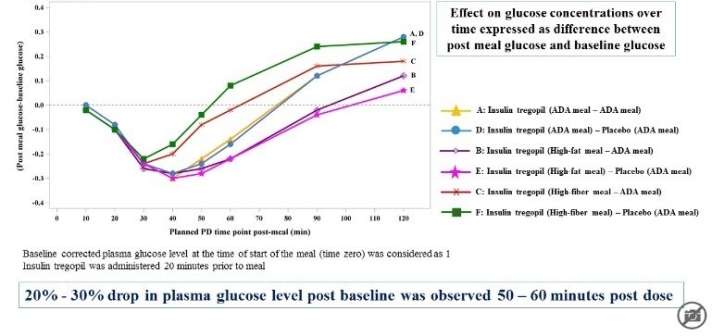
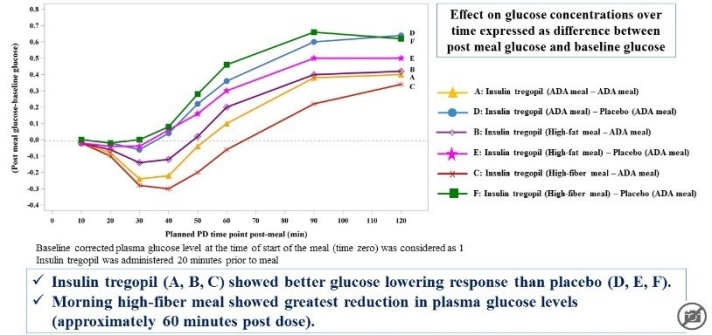

Further Development of Tregopil
Insulin Tregoil is currently undergoing a pivotal Phase II/III study in Type 2 diabetes patients in India.
Insulin Tregopil provides a novel opportunity for effective postprandial control of glucose metabolism through the physiological route of the portal system. In addition, because it can enable early initiation of insulin therapy, this approach holds the potential to protect beta cells and may thereby delay disease progression.
Biocon has persisted and invested in this long development phase for this potentially game-changing oral insulin driven by its strong belief that it has the potential to make a huge difference in the lives of people suffering from diabetes worldwide.


Sir when coming oral insulin for diabetics. pls reply me
LikeLike